DETOUR – the feasibility of immuno OCT
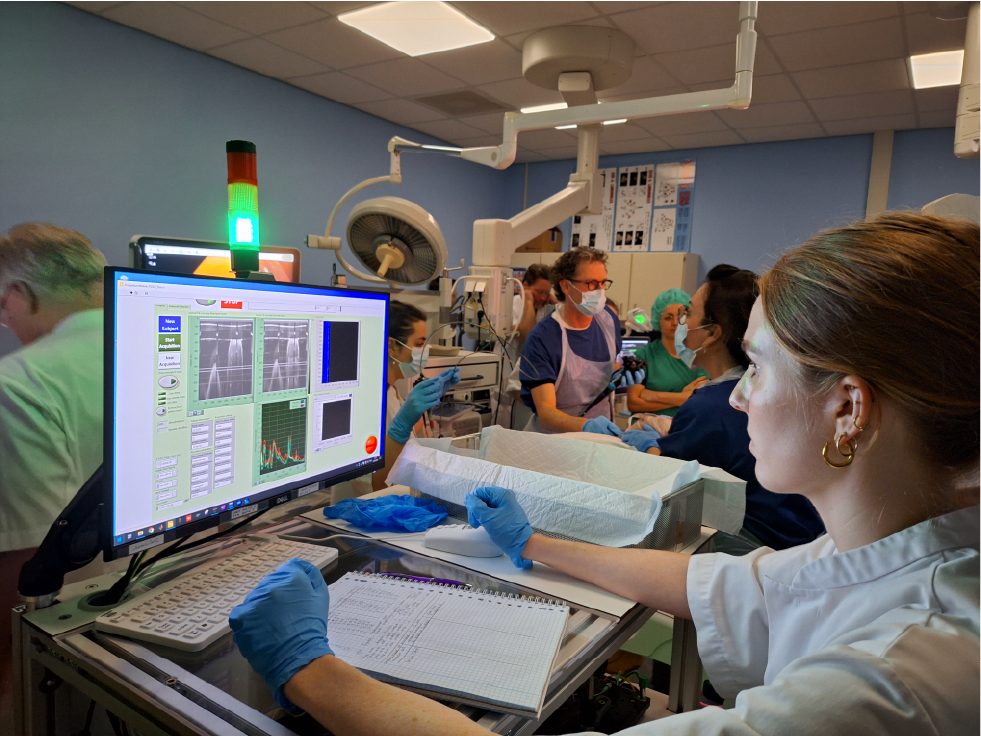
Much of the research done within the OMIG endoscopy is to improve the detection of (pre)malignant lesions using fluorescence molecular endoscopy (FME). There is a need for improved visibility and the possibility of targeted biopsies. In several phase-I and phase-II studies we already proved the feasibility of fluorescent tracers to improve the detection rate of early premalignant lesions. In the DETOUR we will test the feasibility of immune optical coherence tomography (immune-OCT) in combination with fluorescence imaging (NIRF) with our targeted tracer bevacizumab-800CW. The system provides more depth information and can eventually be used without the guidance of the regular endoscopy system. This study is done in cooperation with the faculty of science of the VU Amsterdam (prof. dr. Johannes de Boer) and Green Light Leiden.

Study design
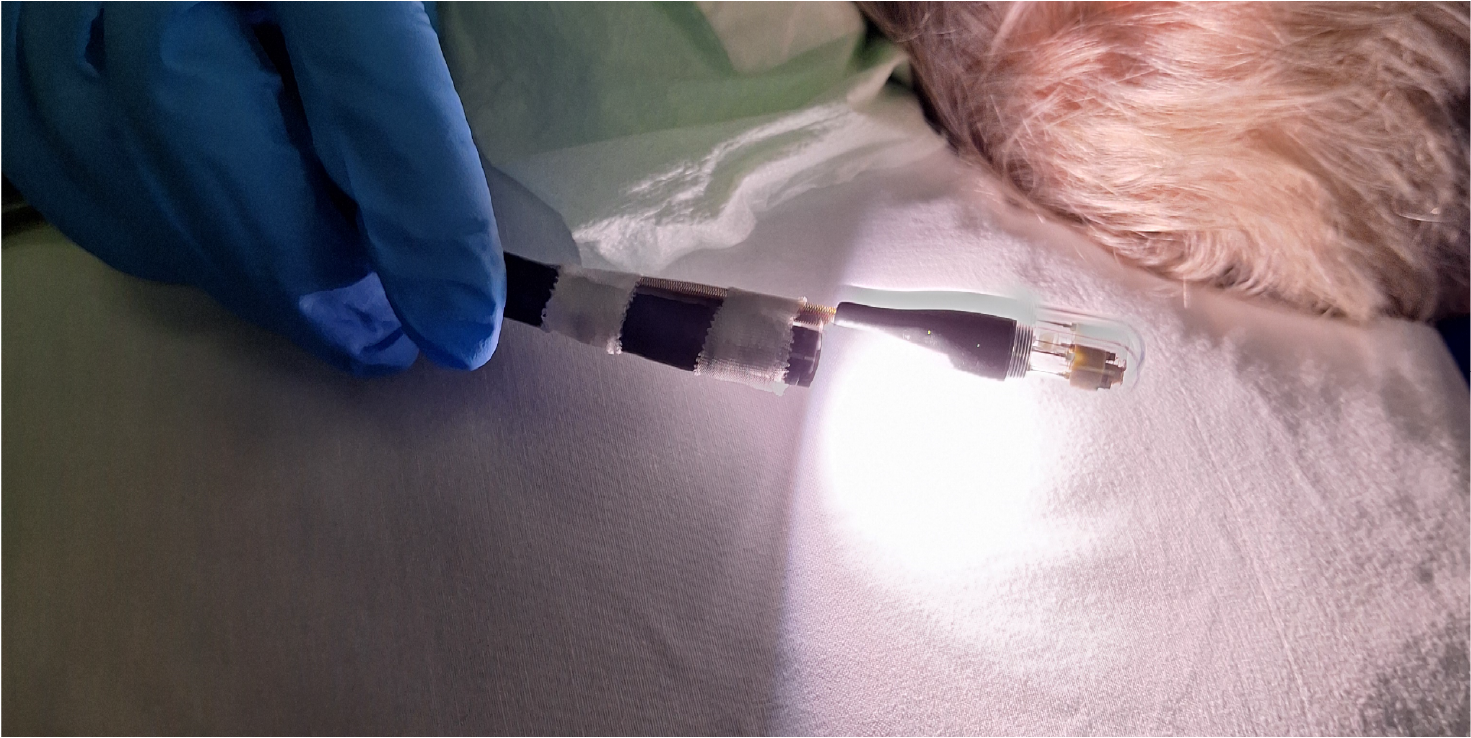
The technique is compiled in a swallowable capsule and can be pulled back using a catheter, allowing easy access to the body without an endoscope. In total 15 patients scheduled for an endoscopic resection will be included. 10 of them will receive an intravenous injection of bevacizumab-800CW of which 5 with esophageal lesions and 5 with colorectal lesions. Another 5 patients with esophageal lesions will receive topical administration of the tracer. After regular endoscopy, using the high-definition white light endoscope (HD-WLE), FME will be applied to inspect suspicious fluorescent areas. The added value of FME in this type of disease was already tested by our group before. Thirdly, immune-OCT will be applied to test the feasibility and validate the system based on HD-WLE, FME and pathology.
Using this research, we can test the feasibility of the immune-OCT capsule to enable easier checkups in patients. The DETOUR will focus on showing the feasibility, larger trials will have to prove its added value in the diagnostic process.