PREDICT – Improving detection and treatment of locally advanced rectal cancer fluorescence endoscopy with nivolumab-800CW and durvalumab-680LT
Colorectal cancer (CRC) is commonly treated with a combination of chemotherapy, radiation, and surgery. Recently, immunotherapy targeting Programmed Death 1 (PD-1) and Programmed Death Ligand 1 (PD-L1) has shown promise in enhancing treatment outcomes. While higher levels of PD-L1/PD-1 are believed to correlate with better responses, predicting how well and when a patient will respond to immunotherapy remains a significant challenge.
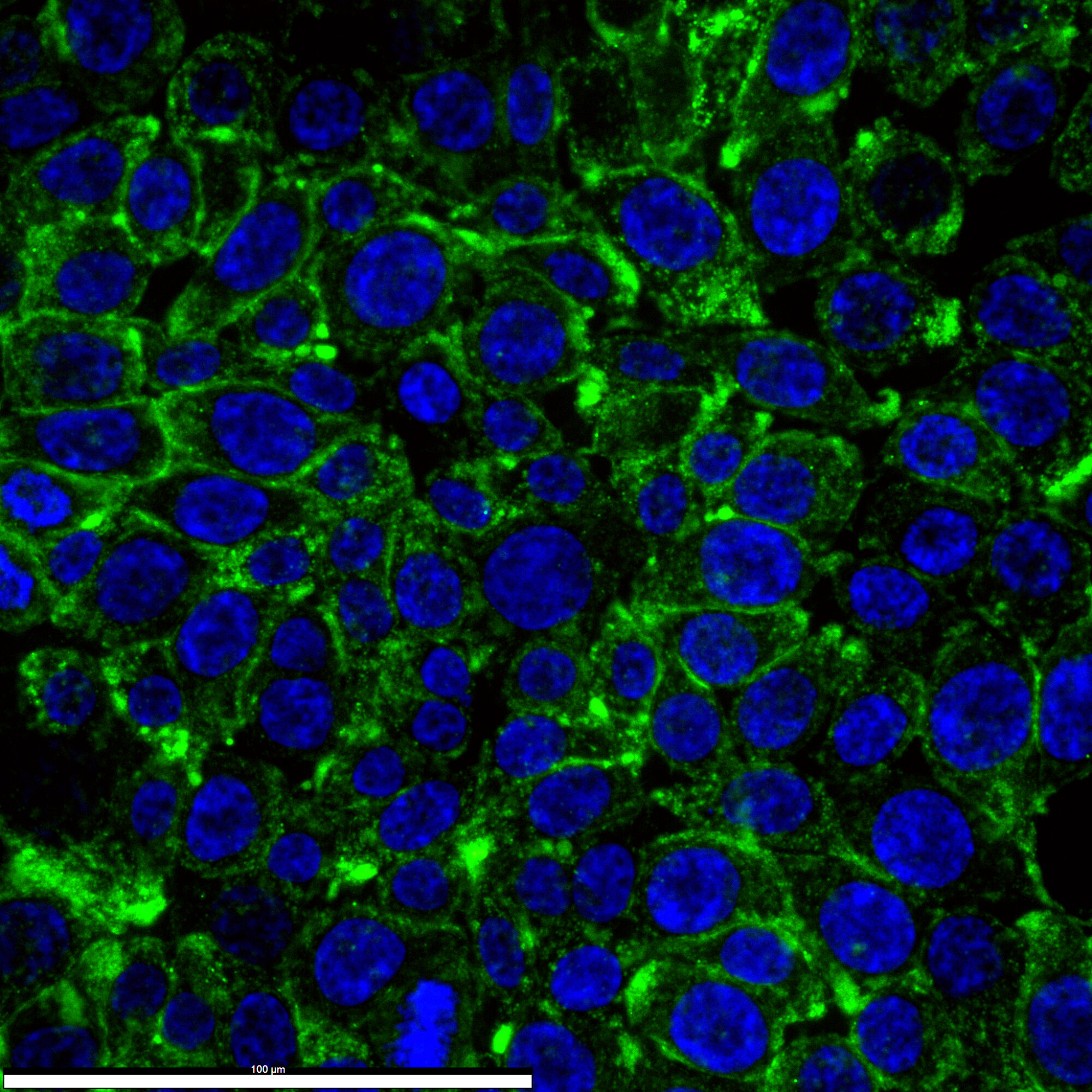
To address this, we employed fluorescently labeled versions of the anti-PD-L1 drug durvalumab (durvalumab-680LT) and the anti-PD-1 drug nivolumab (nivolumab-800CW). This innovative approach enables the direct measurement of PD-1/PD-L1 levels in vivo using a technique called dual-wavelength quantitative fluorescence molecular endoscopy (qFME).
Clinical trial
Eighteen patients with CRC will receive intravenous injections of nivolumab-800CW and durvalumab-680LT three days before undergoing their first qFME procedure. During the procedure, in vivo, fluorescence will be measured and visualized, and biopsies will be collected for ex vivo analysis.
Following the initial procedure, patients will begin chemoradiotherapy (CRT) and return for a second qFME session shortly after completing CRT. Fluorescent signals will be quantified in vivo and ex vivo and correlated with PD-1/PD-L1 expression. Additionally, fluorescence signals from the two time points will be compared, providing new insights into drug distribution, target expression, and changes over time.
