Tracer Development

In collaboration with the Department of Clinical Pharmacy and Pharmacology (CPP) of the UMCG, tracers for clinical trials performed within the OMIG are produced at the GMP facility of the UMCG.
Tracer Development
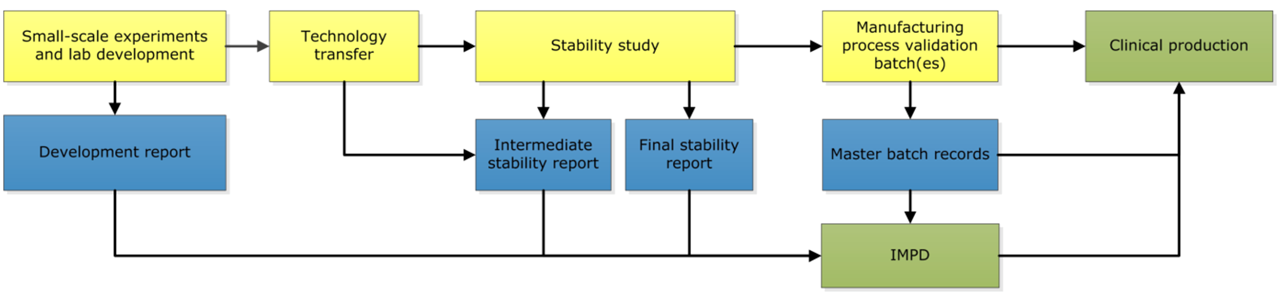
To start a clinical trial with a new fluorescent tracer, the tracer first needs to be developed. The OMIG and CPP work together on these development projects. The CPP is ISO9001 certified, possesses a GMP manufacturing license, and complies with good clinical practice. Initial tracer development is performed at the laboratory unit of the CPP, a ISO15189 accredited laboratory.
Tracer development comprises several phases. First, feasibility testing is performed to determine the feasibility of labelling the desired antibody with one of our IRDyes. Subsequently, the labelling is optimized in the lab and all required quality control (QC)-tests are developed. As soon as this is all achieved, the production process will be transferred to the cleanroom facility.

Tracer production and quality control
Together with the unit of Special Products of the CPP, we produce clinical batches of our fluorescent tracers. The unit of Special Products focuses on innovative short-term projects, especially translation from a lab-setting to the clinical trial phase. The unit has access to class B and C cleanrooms and is equipped with a range of techniques, such as UV-VIS spectrophotometry, and an assortment of Ph. Eur. compendial analysis in order to perform quality control.
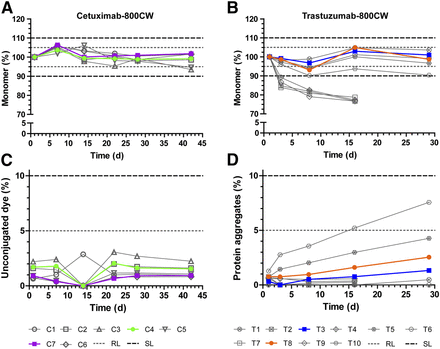
After the development in the laboratory, the first batch in the cleanroom is made to test the production process. Afterwards, this batch is used for a stability study. Together with the involved hospital pharmacists at the CPP, the pharmacists of the OMIG compose the required documentation for the new tracer, such as the IMPD and the product file. Once completed, a clinical batch of this new tracer can be produced and a new clinical trial can start after obtaining ethical approval. Beside new tracers, new batches of already developed tracers are also produced in the unit of Special Products.


Tracers used in our clinical trials
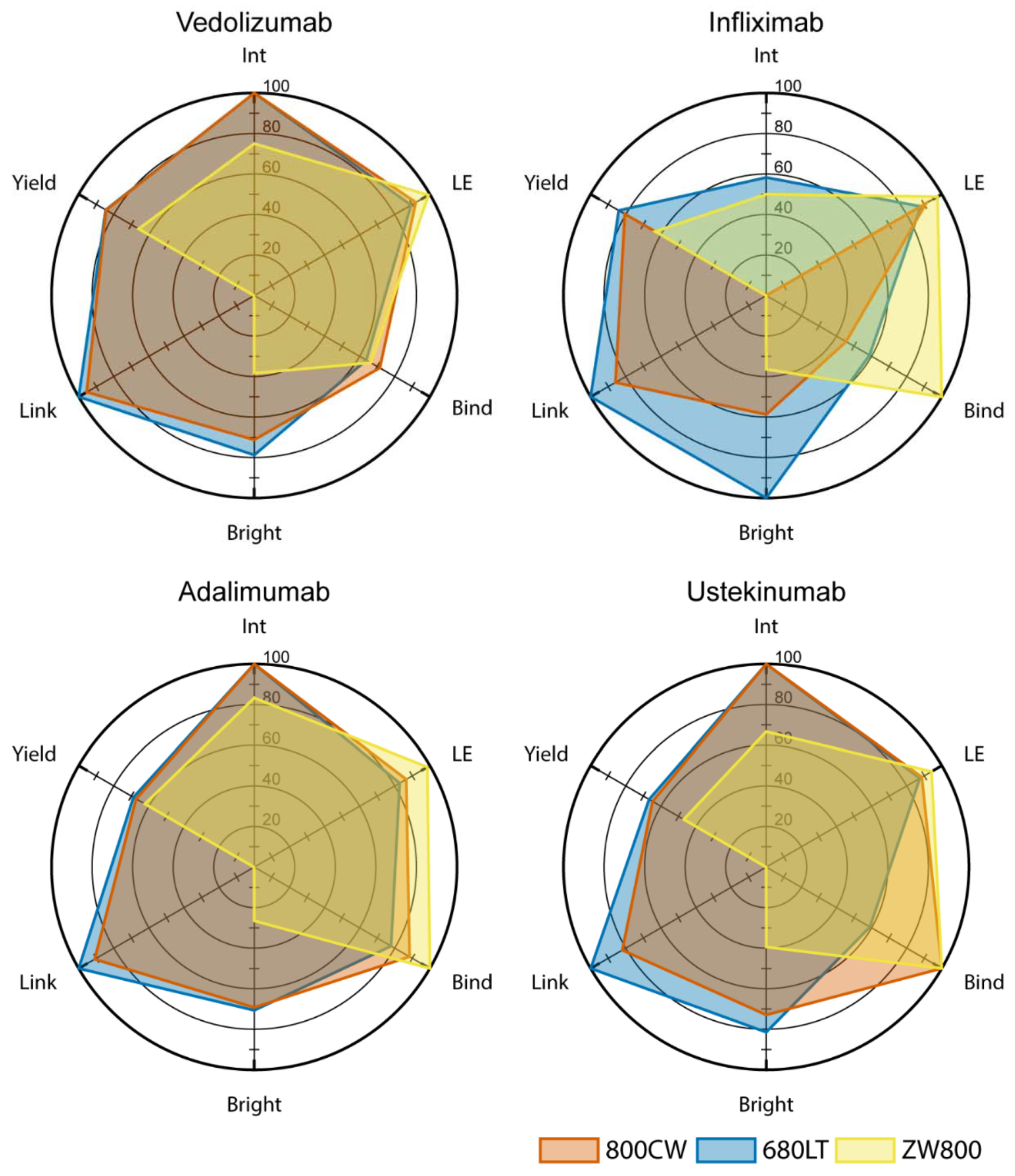
Together with the OMIG, the Unit of Special Products has developed several monoclonal antibodies conjugated to the near-infrared fluorescent dye IRDye 800CW or IRDye 680LT. These antibodies are currently used in clinical trials. The close contact between the clinical researchers and the CPP enables a thorough evaluation of the performance of the tracers and exploration of interesting research opportunities where additional tracers development is needed. This is for example described in a publication where methods for testing and selecting potential antibody-dye conjugated are described.
The tracers currently used in clinical trials are:
- Bevacizumab-800CW
- Vedoluzimab-800CW
- Cetuximab-800CW
- Durvalumab-680LT
- Adalimumab-680LT
- Nivolumab-800CW
- Risankizumab-800CW
- Ustekinumab-800CW
Publications