VIDEO – Quantitative fluorescence molecular imaging of ustekinumab-800CW in inflamed tissue in Crohn’s disease and psoriasis.
Crohn’s disease (CD) and psoriasis (PsO) are autoimmune diseases affecting the gut and skin, respectively, significantly impacting quality of life. Numerous medications, including biologics, are available to treat these conditions, but selecting the most effective drug for an individual patient remains challenging.
Background
While some medications work exceptionally well for certain patients, they may be ineffective for others. Treatment response can only be evaluated after several weeks, during which disease progression may occur if the therapy proves ineffective. Additionally, the efficacy of medications can diminish over time, leading to a loss of response. Therefore, gaining a deeper understanding of how these biologics work is crucial, enabling us to better predict which patients will respond to treatment and which will not.
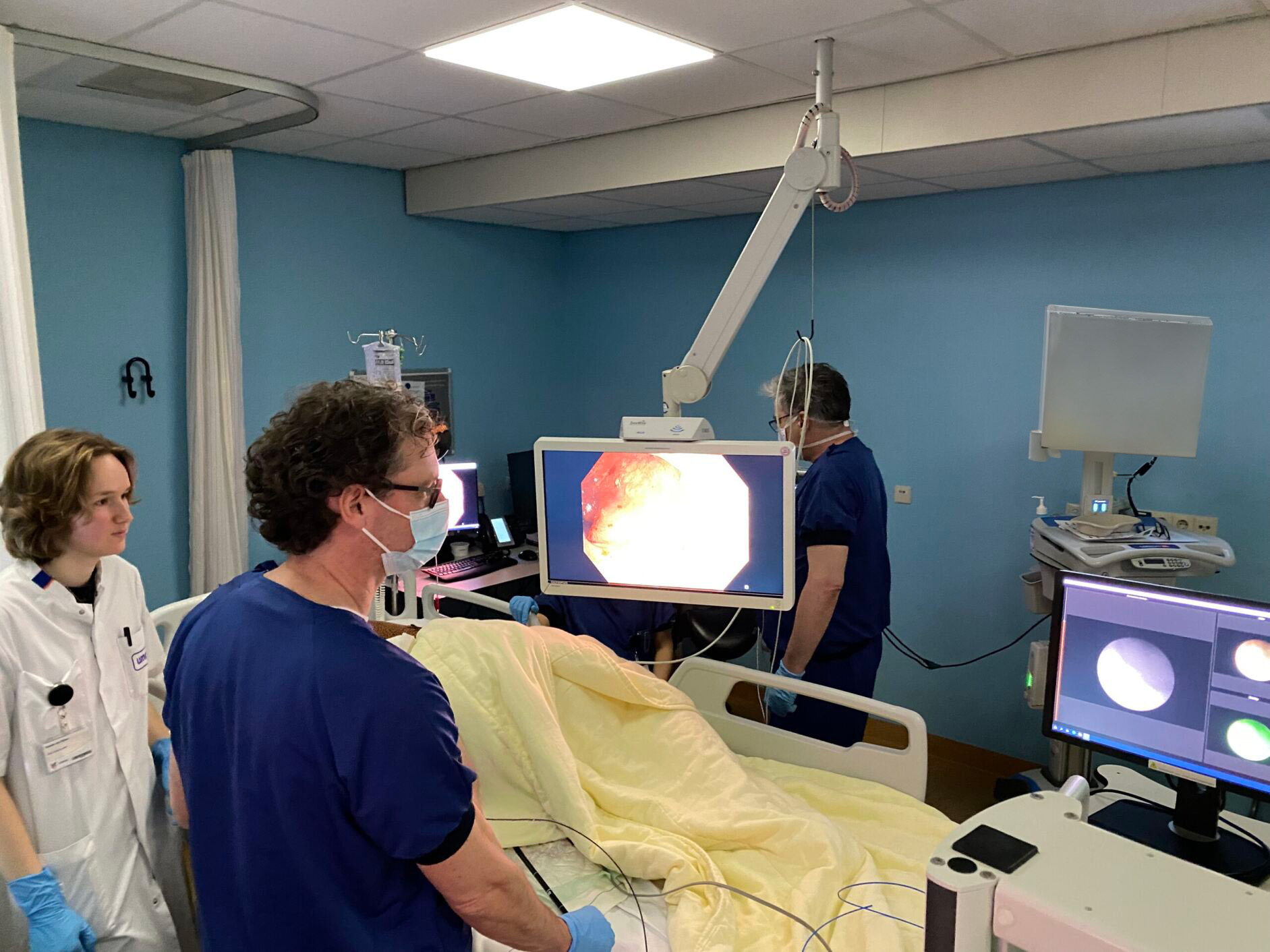
This study aims to investigate the distribution of ustekinumab. To achieve this, ustekinumab has been fluorescently labeled and will be administered intravenously to patients with CD or PsO. By tracing the fluorescently labeled drug within the body, we aim to determine its localization and the cellular interactions it engages in. Ultimately, we hope these insights will enable the prediction of patient response to ustekinumab, paving the way for targeted drug selection and personalized treatment strategies tailored to each patient.
Study design
The current study is a non-randomized, non-blinded, prospective, monocenter safety and feasibility dose-finding study. CD and PsO patients (15 patients in each cohort) will receive an intravenous bolus injection of a sub-therapeutic dose of ustekinumab-800CW 2–4 days before imaging. Imaging will be performed using fluorescence molecular endoscopy (CD) or wide-field fluorescence molecular imaging (PsO). The signals will be quantified using spectroscopy. Additionally, biopsies will be collected for ex vivo analysis to correlate with the in vivo observed and quantified signals. Fluorescence microscopy will be conducted in-house to identify ustekinumab-targeted cells in inflamed and non-inflamed tissue. A second study arm will include 10 additional patients per cohort who are already receiving ustekinumab therapy, preferably those who also participated in arm 1.
